SMArtCARE Case Study:
Real-World Data Collection Enables Evaluating Safety and Effectiveness of Treatments for Spinal Muscular Atrophy
This exciting initiative combines collecting real-world patient data by neurologists, clinicians and patients to enable clinical research and engagement of spinal muscular atrophy patients receiving treatment.
SMArtCARE is a multi-year joint initiative of neurologists, paediatricians, and patients with spinal muscular atrophy (SMA). Spinal muscular atrophy refers to a group of rare genetic diseases resulting in muscle wastage and weakness. Symptom onset can be seen in both children and adults but the most severe form typically presents in children under 18 months old. Until recent treatments survival past childhood was unusual.
Its imperative is to improve patient outcomes and ensure that specific spinal muscle atrophy treatments are accurately assessed for both safety and effectiveness post authorization.
From Clinical Trials to Real-World Dispersed Data Collection
While targeted treatments are developed in pre-clinical and clinical settings, SMA sufferers also have notably high treatment variance but collecting this data during routine care visits over the long-term can be difficult.
Historically, there have been different data repositories, and inevitably there are challenges with aligning data formats, inconsistent coding and fragmentation. Ensuring data consistency and accuracy to support research becomes very challenging. Data fragmentation, with some information in one system and different but related information in another system, makes it hard to evaluate individual patient outcomes. Without this consolidated data, it is difficult to accurately capture the variance in treatments and real-world outcomes across different patients and geographies.
SMArtCARE concluded that a single disease-specific patient registry would address these data collection challenges. SMArtCARE’s vision enabled creation of the spinal muscular atrophy disease registry for longitudinal data collection on all available SMA patients, providing an invaluable dataset for efficacy research.
SMA Data Collection Objectives & Parameters
Once SMArtCARE established the need to securely and accurately collect research-ready data from both clinicians and patients, more requirements came into focus. Considerations included the variety of treatments and outcomes, and the collection of data across multiple clinical centres in different countries.
To create a robust structured data set for SMA research required the following:
- Capture and benchmark existing and new SMA treatments, quality of life and care regimes.
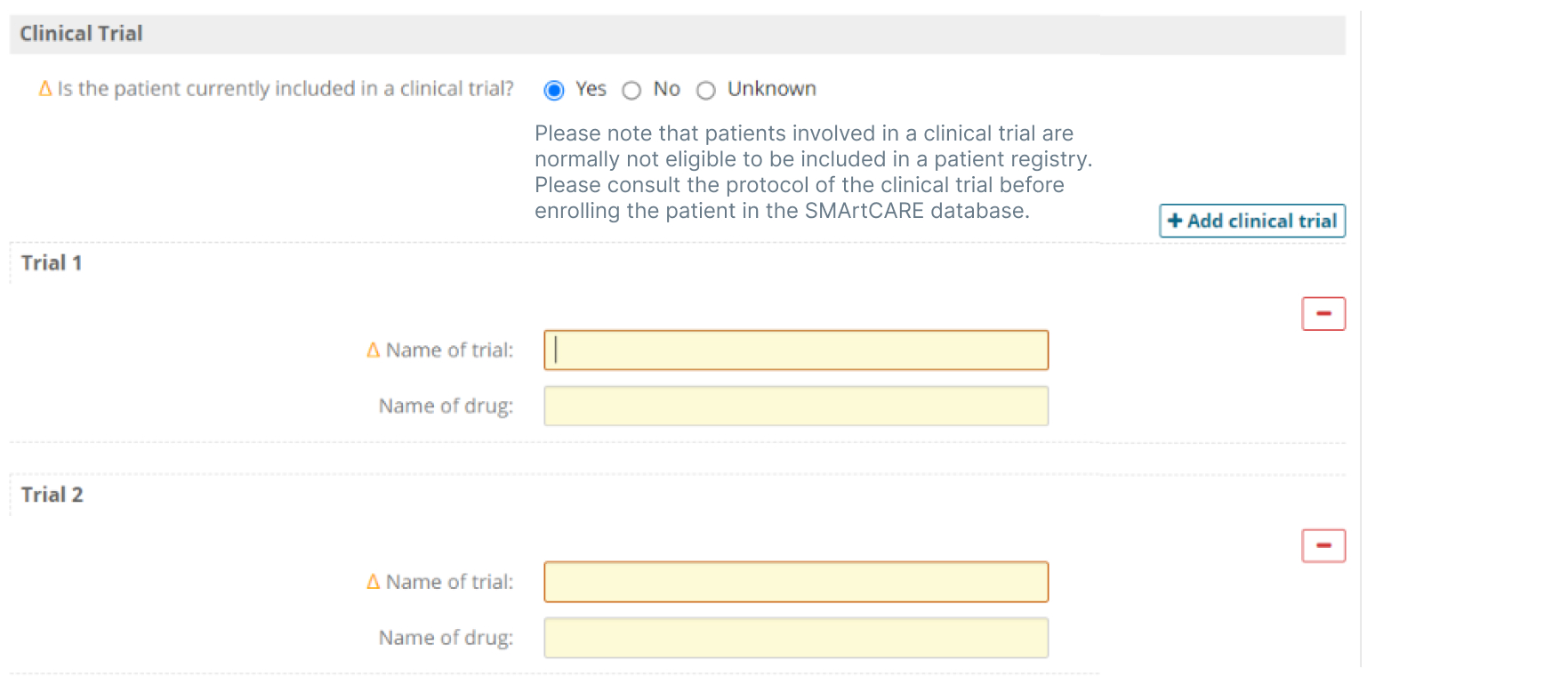
- Provide a facility to record previous randomized clinical trial(s), including for example, both the trial name and drug being assessed.
- Ensure patient confidentiality, consent and data security with a robust user management system and secure software technology infrastructure.
- Supports both English and German speakers. This was especially important for patients and to enable the participation of international centers.
- Avoid data fragmentation by combining data from both patients and clinical teams to create a dataset suitable for research.
SMA clinicians suggested the following requirements:
- Enable clinical data collectors to reliably and quickly capture scheduled and unscheduled visits and assessment information such as age, weight, pulmonary events and vaccinations.
- Record treatment efficacy as there was a strong need to monitor all treated and untreated SMA patients in a real-life environment to optimize efficacy. This also includes capturing adverse events that could potentially be attributed to new treatments.
To provide a rounded picture of patient outcomes, it was important to engage patients and carers in data collection to support the following requirements:
- Support Patient Recruitment -SMArtCARE wished to engage patients by providing information that encourages and demonstrates the patient role in disease learning and management.
- Engage Patients and Carers: Provide personalized health statistics and trends to help patients understand and adhere to treatments, and to assist them manage their well-being.
Collaborative Bespoke Registry Development Based on Robust eHealth Data Collection Platform
With the above requirements in mind, SMArtCARE and OpenApp took the following approach. A cloud-based secure patient registry platform, Clinical Insight, was selected to collect and record disease experiences and parameters from clinicians and patients/carers. The secure, GDPR compliant platform enables the development of a longitudinal dataset suitable for research.
Designing SMA-Specific Data Collection
Working collaboratively with SMArtCARE, OpenApp configured Clinical Insight to incorporate SMA specific data sets, advising on logical data structures, optimising user experience and implementing intuitive system workflows. Completed over a series of multi-disciplinary workshops with SMArtCARE, the team developed:
- Data validation rules– to ensure consistent data quality and completeness.
- Clinical data set – to capture a comprehensive record of patient demographics, history, visits and treatments. For example, patient information was categorised into enrolment, baseline and visitation forms to allow both baseline and longitudinal patient data to be reflected.
- Summary SMA Dashboard – a visualization of data for easier navigation of collected data e.g. timeline of the important aspects of a patient’s history.
Creating SMA Clinical Workflows
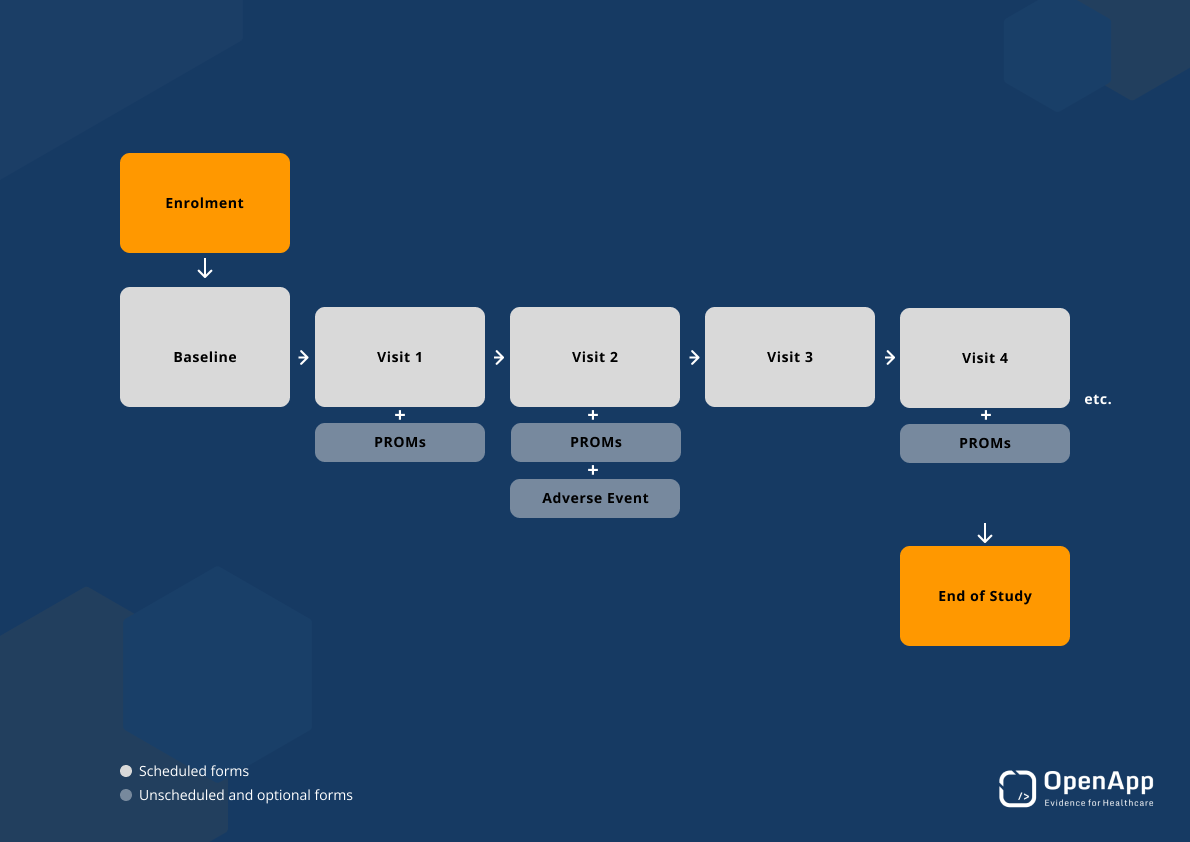
- Clinical workflows were configured to accommodate clinicians’ needs by prompting and offering appropriate forms including consent and enrolment, baseline electronic case report forms (eCRF), encounter capturing, patient reported outcome measures (PROMs) and end of study forms.
- Prototypes allowed the SMArtCARE team to explore and test how clinicians would experience data capture in practice aiming for minimum interference by incorporating data collection into the typical SMA clinical setting/visit.
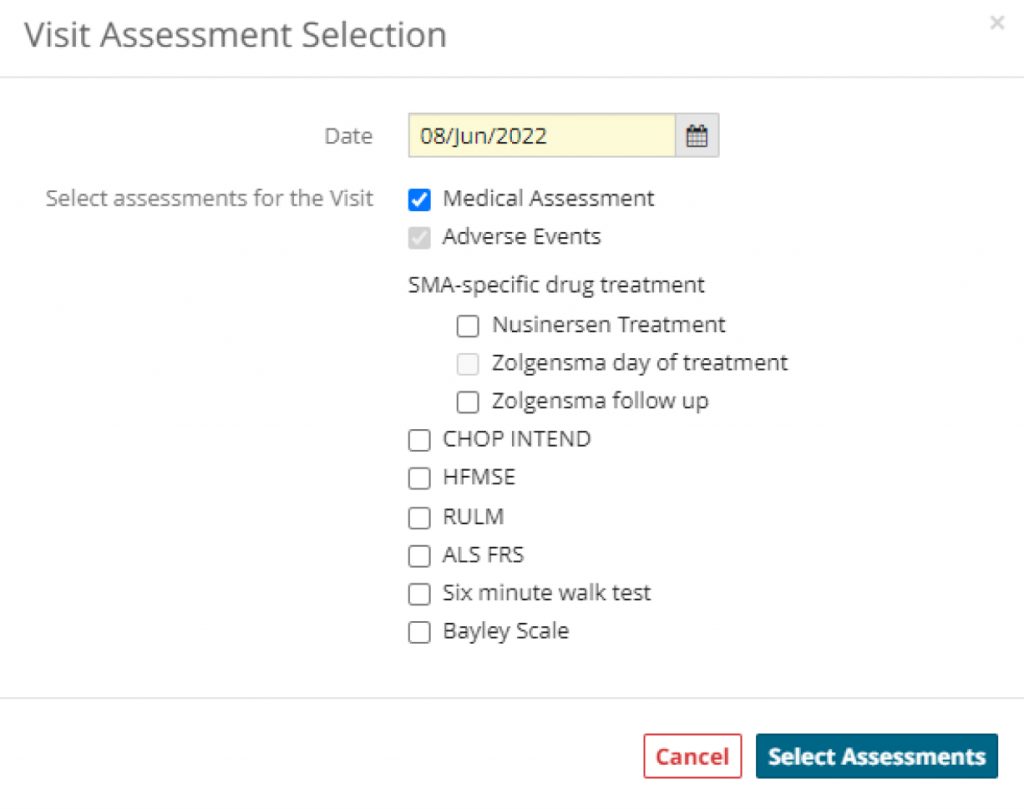
- Assessment selection tool allows the clinician / investigator to define which assessments, such as physical therapy instruments or PROMS will be performed at a specific patient visit.
Thousands of Patients Across 70 Medical Centers in 4 Countries
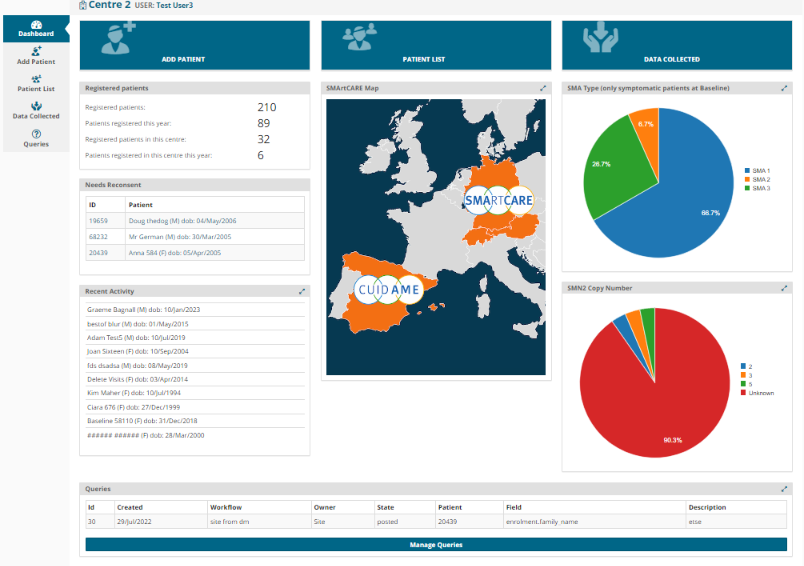
Originally launched in 2019 across ~50 medical centers throughout Germany, Austria and Switzerland, the SMA disease registry continues to grow with ~2000 patients across over 75 centers currently. The platform expanded to include Spain in 2021.
Fully compatible with GDPR, the cloud-based software stores thousands of structured data records contributed by both clinical team members as well as from patients/carers.
A dedicated patient portal which has a dashboard giving individual patients a view of appropriate clinical information from their own registry records.
Using Real-World Data Collection for Efficacy Monitoring
In 2022, the SMArtCARE registry was designated as the mandatory data collection vehicle for monitoring the efficacy of Zolgensma® and Spinraza® (Nusinersen) by Germany’s Federal Joint Committee(GBA), the body responsible for defining clinical practice. The registry also provided systematic data collection for evaluation of Nusinersen prior to European approval.
Future Flexible
The platform is very configurable and has been modified many times to track new treatments for the SMA community. This has allowed the addition of relevant endpoints for treatments and the addition of functionality as the clinical requirements changed. We expect that there may be more platform changes to meet future goals, and updated requirements.
Learn More About the SMA Disease-Specific Registry
- Learn more about the research publication using SMA disease registry data
- Enquire about participating in SMArtCARE data collection, please contact SMArtCARE@uniklinik-freiburg.de
- Learn more about Rare Disease Patient & Clinical Registries here
Latest News
Real-World Data Collection Enables Evaluating the Safety and Effectiveness of Treatments for Spinal Muscular Atrophy
This exciting initiative combines collecting real-world patient data by neurologists, clinicians and patients to enable clinical research and engagement of spinal muscular atrophy patients receiving treatment.
SMArtCARE is a multi-year joint initiative of neurologists, paediatricians, and patients with spinal muscular atrophy (SMA). Spinal muscular atrophy refers to a group of rare genetic diseases resulting in muscle wastage and weakness. Symptom onset can be seen in both children and adults but the most severe form typically presents in children under 18 months old. Until recent treatments survival past childhood was unusual.
Read MoreChildrens Tumor Foundation Innovative Patient Reported Patient Registry Platform
The NF registry is a one of a kind project, where the OpenApp team worked in collaboration with CTF to develop and support a platform that meets their requirements, as a secure and effective tool to empower NF patients and their caregivers. A dedicated registry is the most efficient way to raise awareness/advocate for NF, expand the NF community, and connect to help end NF.
Read MoreWhy You Need A Software Maintenance Management Plan
Combining the development efforts and the ongoing maintenance of your software is key to ensuring that you have a robust and constantly improving and evolving solution.
Having a software maintenance plan is just as important as the initial development. Professionally managed maintenance allows for the continual improvement and adaptation to changing business needs and technological advancements.
Read MoreIQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry dedicated to delivering actionable insights. Learn more at www.iqvia.com.

Copyright © 2024 | Privacy Policy | Information Security Policy
OpenApplications Consulting Ltd. Registered in Ireland No. 355595






