Patient Registry Software
Collect a Disease-specific, Research Ready, Real-World Dataset
Collect disease experience, lab data, care management and interventions across clinical domains to enable research studies and for longitudinal research. OpenApp’s proven, secure GDPR-compliant disease registry software is a fully managed cloud-based flexible platform. Collect and safely store, hundreds of data points from thousands of patient forms in clinical and other patient settings, for a rare or low prevalent disease, or for a product-specific dataset.
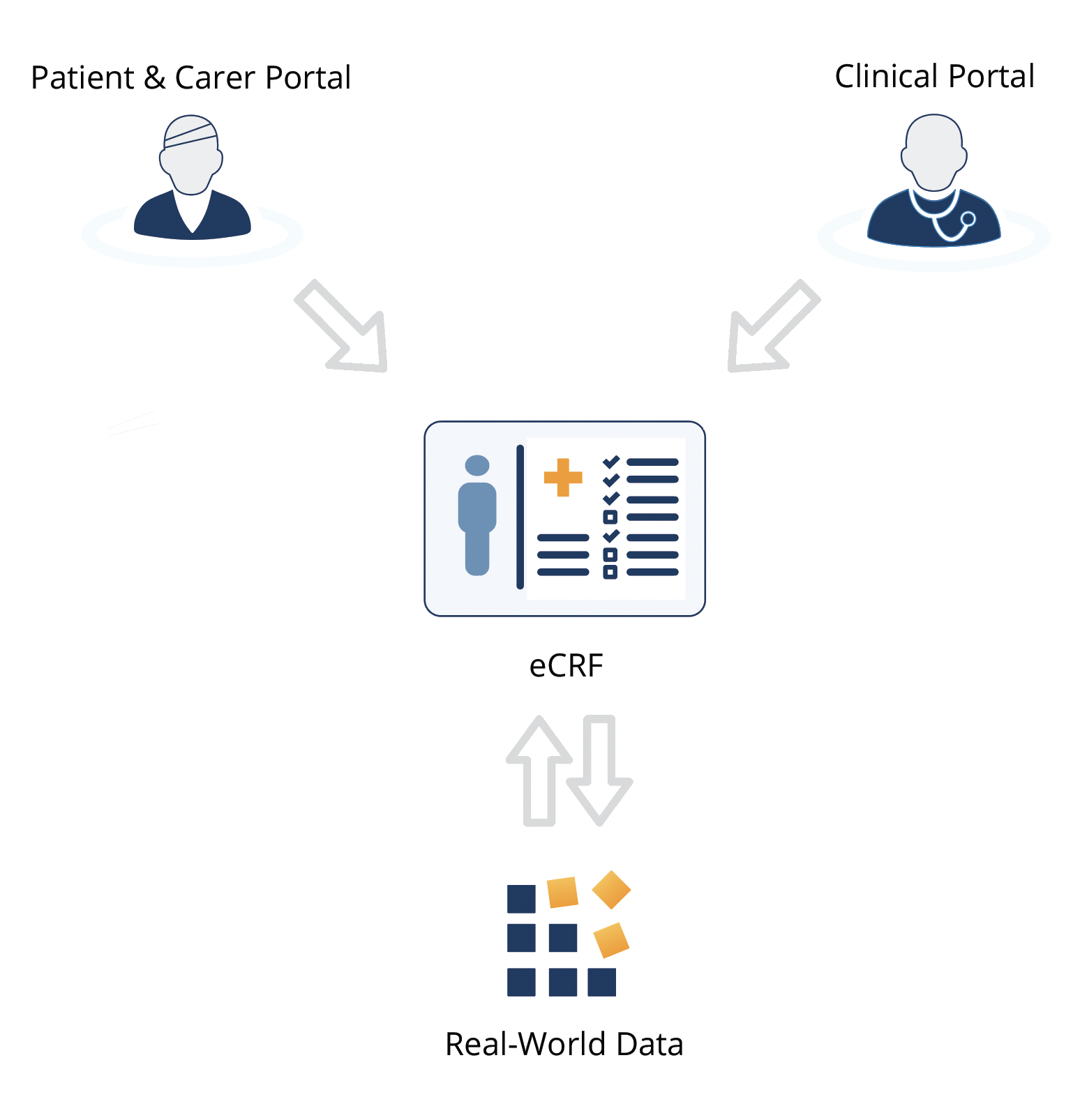
Core Patient Registry Features
- Collect and Manage Flexible Patient Data Sets
- Examples include demographic data, medical history, genetic profiles, diagnostic report, medication, adverse events, and more.
- Suitable for Natural History and Observational Studies
- Migrate Existing Data Sources
- Multilingual Options
- GDPR Compliant Platform
Clinical Reported Data for Healthcare Professionals
- Electronic Case Report Forms (eCRF)
- Enrollment and Baseline Forms
- Encounter and Follow-up Forms
- Patient Reported Outcome Measures (PROMs)
- Configure Access By Clinical Centre
- Healthcare Professional Dashboards
Patient Reported Data for Patient Organizations
- Participant (Self-)Enrollment
- Customise Consent. For example by registry participation, trial contact, contribute anonymously to research
- Scheduled Surveys
- Segment by Patient Cohort
- Email Communications by Cohort
- Registry Manager Dashboards
Research Study Manager Features
- Audit Trails
- Discrepancy Notes
- Freeze and Lock Data
- Report Query Builder
- Adhoc and Pre-Configured Query Reports
- Data Export (.csv, excel, CDISC)
Latest News
Real-World Data Collection Enables Evaluating the Safety and Effectiveness of Treatments for Spinal Muscular Atrophy
This exciting initiative combines collecting real-world patient data by neurologists, clinicians and patients to enable clinical research and engagement of spinal muscular atrophy patients receiving treatment.
SMArtCARE is a multi-year joint initiative of neurologists, paediatricians, and patients with spinal muscular atrophy (SMA). Spinal muscular atrophy refers to a group of rare genetic diseases resulting in muscle wastage and weakness. Symptom onset can be seen in both children and adults but the most severe form typically presents in children under 18 months old. Until recent treatments survival past childhood was unusual.
Read MoreChildrens Tumor Foundation Innovative Patient Reported Patient Registry Platform
The NF registry is a one of a kind project, where the OpenApp team worked in collaboration with CTF to develop and support a platform that meets their requirements, as a secure and effective tool to empower NF patients and their caregivers. A dedicated registry is the most efficient way to raise awareness/advocate for NF, expand the NF community, and connect to help end NF.
Read MoreOpenApp supports Metachromatic Leukodystrophy Disease MLD Research
To celebrate Rare Disease Day 2023, which takes place on the 28th of February, we are changing our colours and showing our support for the Metachromatic Leukodystrophy Disease (MLD) Support Association UK. OpenApp have been working MLD support Association UK for many years under our Rare100 CSR scheme, a Corporate Social Responsibility (CSR) initiative run in 2018.
Read MoreIQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry dedicated to delivering actionable insights. Learn more at www.iqvia.com.

Copyright © 2024 | Privacy Policy | Information Security Policy
OpenApplications Consulting Ltd. Registered in Ireland No. 355595




